A rigorous process
The process for biosimilar medicine development involves rigorous analytical studies to establish a comprehensive understanding of the similarity of the biosimilar to the reference product. Assessments of toxicity and clinical studies are also used to further establish similarity. Ultimately, the goal is to demonstrate that there are no clinically meaningful differences between the reference product and the biosimilar based on the findings from all of these studies.
Building on the established clinical profile of the reference biologic.
Biosimilar development requires substantial time and financial investment
Development of biosimilar medicines begins with substantial investment in the specialized infrastructure, expertise, and technology required to create the product, verify that it is biosimilar, and ultimately to maintain quality production.
Drug development comparison
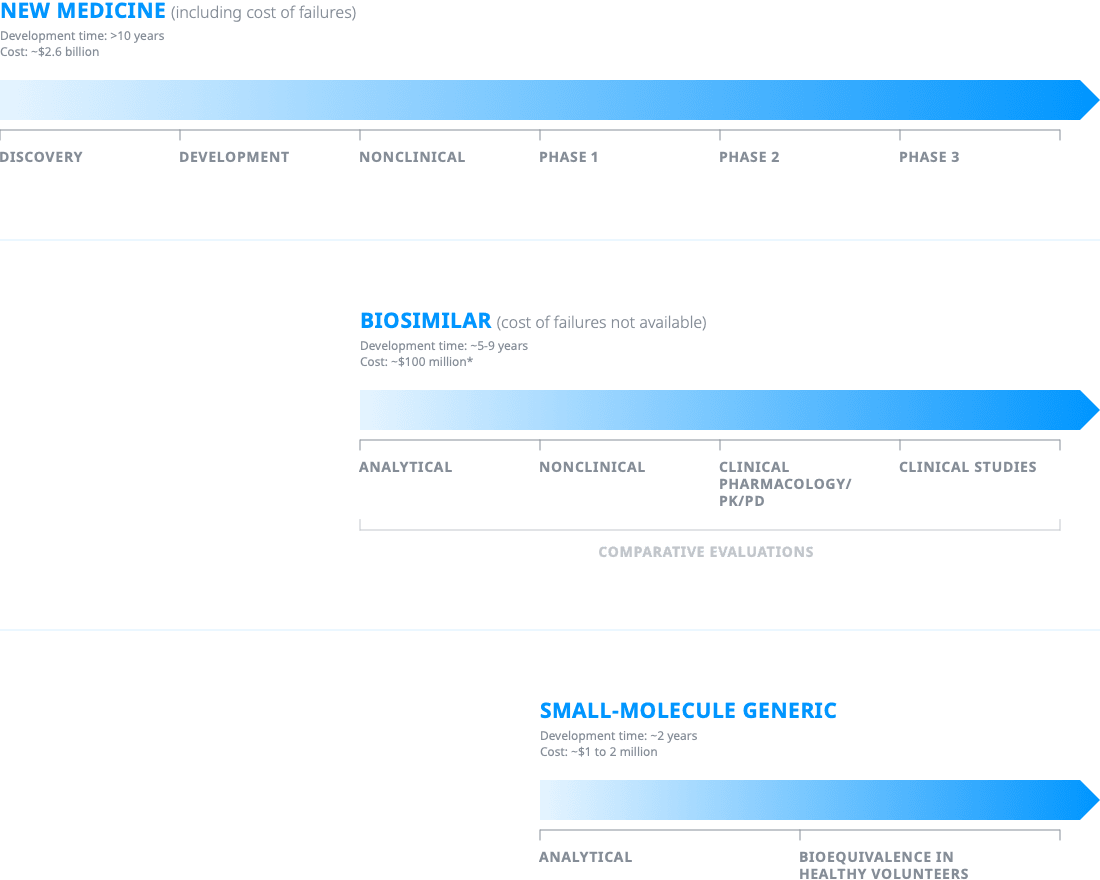
*Not including regulatory fees.

The development processes for biologics and biosimilars are considerably more rigorous than the development process for small-molecule generics.