Confidently integrating biosimilars into practice
Biosimilars have layered additional complexities into the traditional evaluation process used by Pharmacy and Therapeutic (P&T) committees in considering a new product for formulary inclusion. To ensure efficient implementation, a thoughtful assessment of the strategies and tactics related to an institution’s operational considerations is required.
P&T evaluations include several key aspects (including scientific review, financial and coverage assessment, and operations). Thoughtful considerations of these aspects will help build a thoughtful implementation strategy. Clear timelines, defined roles and responsibilities, and a careful assessment of resources will help to ensure smooth implementation and strategy execution.
Strategic considerations for P&T evaluation

OPERATIONAL
CONSIDERATIONS
PHARMACY-DRIVEN IMPLEMENTATION STRATEGY
Supported by defined timelines, roles, and responsibilities, as well as necessary resources for execution at a given institution
Operational enablement of the biosimilar implementation strategy
Clear communication of the biosimilar initiative and implementation strategy should be maintained across stakeholders throughout the medication-management continuum.
Operational considerations from 4 key operational areas may be included in the implementation strategy.
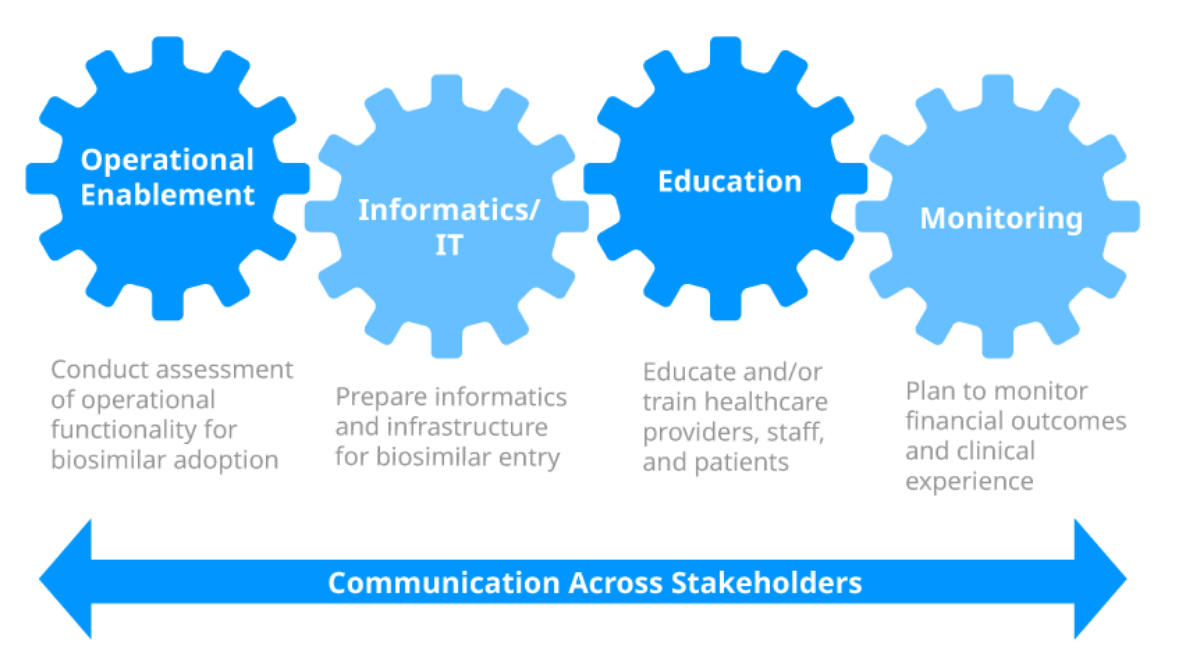
Click below to access a series of Informational Videos for a deeper dive into these 4 key operational areas.