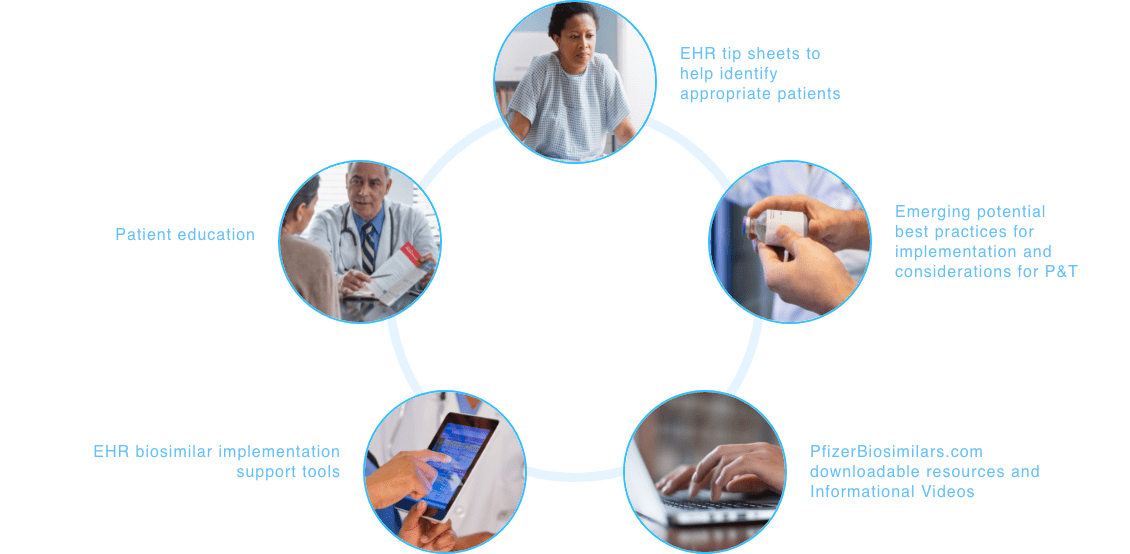
Making your patients’ support needs a priority. Together.
At Pfizer Oncology Together™, patient support is at the core of everything we do. From helping to identify financial assistance options to connecting patients to resources for emotional support, your patients’ needs are our priority.
PfizerOncologyTogether.com