Confidently Integrating Biosimilars Into Practice
Biosimilars have layered additional complexities into the traditional evaluation process used by Pharmacy and Therapeutic (P&T) committees in considering a new product for formulary inclusion. To ensure efficient implementation, a thoughtful assessment of the strategies and tactics related to an institution’s operational considerations is required.
P&T evaluations include several key aspects (including scientific review, financial and coverage assessment, and operations). Thoughtful considerations of these aspects will help build a thoughtful implementation strategy. Clear timelines, defined roles and responsibilities, and a careful assessment of resources will help to ensure smooth implementation strategy execution.
Strategic Considerations for P&T Evaluation


Operational Enablement of the Biosimilar
Implementation Strategy
Clear communication of the biosimilar initiative and implementation strategy should be maintained across stakeholders throughout the medication-management continuum.
Operational considerations from 4 critical elements may be included in the implementation strategy.
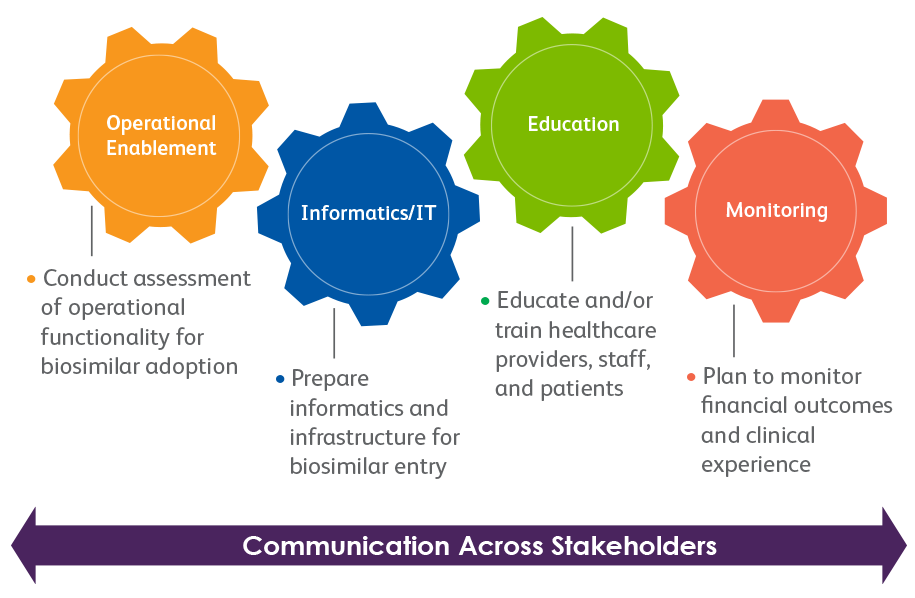

The application of traditional formulary and practice management tools and principles, coupled with key concepts that have been identified as a potential best practice for a P&T committee’s evaluation of a biosimilar for formulary inclusion, may aid in the appropriate adoption, and safe and effective use, of biosimilars in clinical practice.
For a deeper dive into these 4 key operational areas to confidently integrate biosimilars into practice, click this link to download a digital brochure titled Biosimilar Implementation: Potential Best Practices and Other Considerations.


